28 January 2021
PsoProtectMe survey reveals new findings on shielding behaviour during COVID-19 pandemic
Shielding behaviour in people with psoriasis or a joint condition during the COVID-19 pandemic differs by treatment type.
New findings recently published in the British Journal of Dermatology show that shielding behaviour in people with psoriasis or a joint condition (such as psoriatic arthritis) during the COVID-19 pandemic differs by the type of treatment the individual is receiving for their condition.
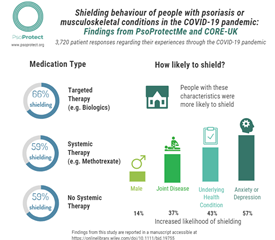
The researchers found that a greater proportion of people who were receiving targeted treatments, such as biologics, reported shielding compared to those receiving no treatment or traditional tablet immunosuppressants.
Since they may be less exposed to the virus, this may explain why people taking biologics have been reported to have a lower risk of severe COVID-19 infection in other studies.
These findings are based on data collected through the global PsoProtectMe survey for people with psoriasis, and the UK-based survey for people with joint conditions, CORE-UK.
Here is a short summary of the research findings:
Public health measures such as social/physical distancing were introduced early in the pandemic to limit the spread of COVID-19. Stricter measures referred to as ‘shielding’ (not leaving home and minimising all face-to-face contact) were recommended in groups of people at higher risk of severe infection. Many people taking drugs that affect the immune system to treat psoriasis or a joint condition (e.g. rheumatoid arthritis) were advised to reduce their risk of developing COVID-19 by shielding. We wanted to find out what factors influenced how much (or strictly) this advice was followed.
To try and answer this important question, we invited people with psoriasis or a joint condition (whether or not they have had COVID-19) to provide information about whether they shielded in the pandemic. We collected this information through a series of structured questions in web-based surveys for people with psoriasis (PsoProtectMe) or a joint condition (CORE-UK). The surveys also included questions about mental health (anxiety and depression) during the pandemic.
Three thousand, seven hundred and twenty people (2,869 with psoriasis and 851 with a joint condition from 74 countries completed the PsoProtectMe and CORE-UK surveys between May and September 2020 (average age 49 years; more women [2,546, 68%] than men [1,174, 32%]). One thousand four hundred and twenty-one participants (38%) reported taking medicines that affect the immune system to treat their psoriasis or joint disease – either targeted treatments such as ‘biologics’ (924 patients, 25%) or traditional immunosuppressants such as methotrexate (497, 13%).
A greater proportion of people who were receiving targeted treatments reported shielding compared to those receiving no treatment or traditional tablet immunosuppressants. As expected, owing to public health guidance, individuals with other health problems were more likely to shield. Individuals were also more likely to shield if they were male, had joint disease, and signs of anxiety or depression.
In this study, people taking targeted treatments such as biologics for their psoriasis or joint disease were more likely to shield. Since they may be less exposed to the virus, this may explain why people taking biologics have been reported to have a lower risk of severe COVID-19 infection in other studies.
End of summary
You can read the full paper here.
If you have psoriasis yourself, you can report your experiences of life during the COVID-19 pandemic (whether or not you have had symptoms of COVID-19) here.
If you are a healthcare professional treating patients with psoriasis, please continue to report cases of suspected or confirmed COVID-19 infection in your patients here.